

Mass spectrum
A mass spectrum will usually be presented as a vertical bar graph, in which each bar represents an ion having a specific mass-to-charge ratio (m/z) and the length of the bar indicates the relative abundance of the ion.
Relative atomic mass

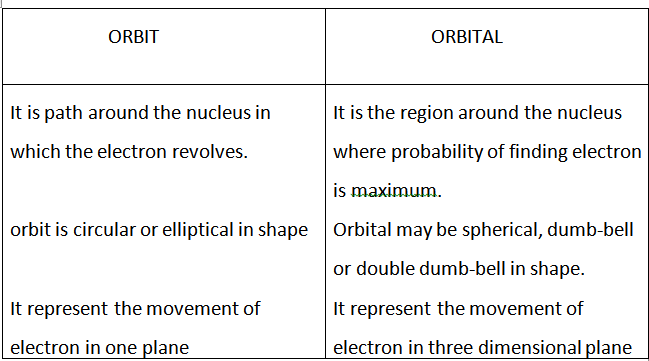
Shapes of orbitals
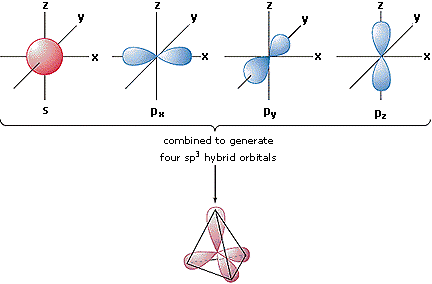

QUESTIONS
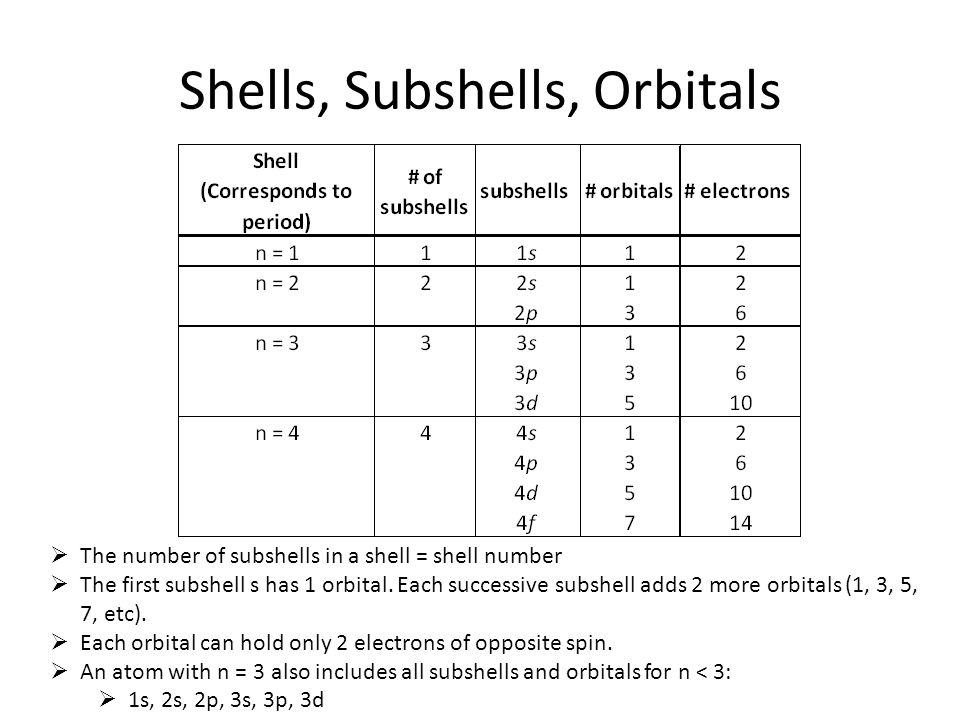
1.How many electrons can be kept in a. 2s sub-shell b. 3d sub-shell
2.Why did Rutherford only take gold foil to bombard alpha rays?
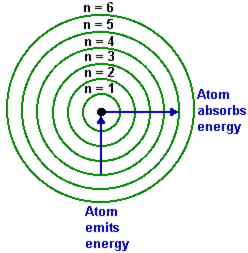
3.Draw a sketch diagram showing the relative energies of the 1s,2s,2p,3s,3p,4s and 4p sub-shells.
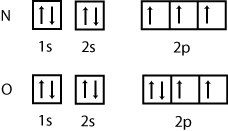



2.Write the electronic configuration of the element X.


















































.png)


No comments:
Post a Comment