| Ernest Rutherford |
RUTHER FORD' S ALPHA PARTICLE SCATTERING EXPERIMENT

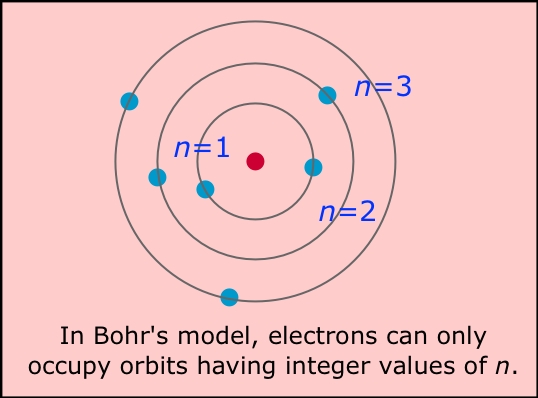
Rutherford's atom model
 |
| Niels Bohr |
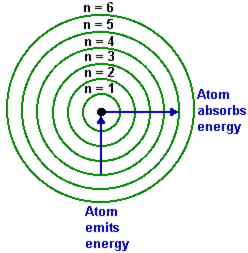
Shapes of orbitals
QUESTIONS
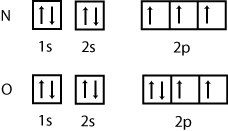
1.How many electrons can be kept in a. 2s sub-shell b. 3d sub-shell
2.Why did Rutherford only take gold foil to bombard alpha rays?
3.Draw a sketch diagram showing the relative energies of the 1s,2s,2p,3s,3p,4s and 4p sub-shells.
LASER IS Light amplification by stimulated emission of radiation.
Luminescence
The electrons in some substances absorb energy then emit light. This is called luminescence.


Atoms and Electrons
http://www.mhhe.com/physsci/chemistry/carey/student/olc/ch01atoms.html
http://www.bbc.co.uk/schools/gcsebitesize/science/add_aqa_pre_2011/atomic/atomstrucrev1.shtml














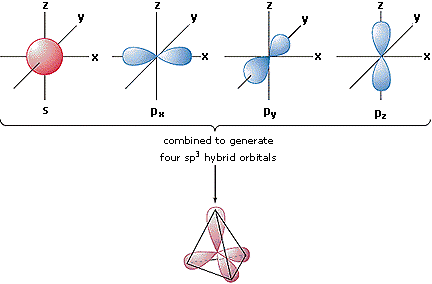
















No comments:
Post a Comment