
Properties of the Alkaline Earth Metals

FIRST IONIZATION ENERGY OF GROUP 2 METALS
First ionization energy decreases going down Group 2Table of physical data
Element
|
Proton
number |
Symbol
|
First
ionisation energy (kJ/mol) |
Beryllium |
4
|
Be
|
900
|
Magnesium |
12
|
Mg
|
738
|
Calcium
|
20
|
Ca
|
590
|
Strontium.
|
38
|
Sr
|
550
|
Barium
|
56
|
Ba
|
503
|
EXTRA QUESTIONS.
1.
State Mendeleev’s periodic law.
2.
State Modern periodic law
3.
Prove that Li Na K belongs to triads.
4.
Explain Mendeleev’s periodic table.
5.Explain
Modern periodic table.
6.Why
K is more reactive than Na?
7.Why
carbon ,silicon have high melting and
boiling points.?
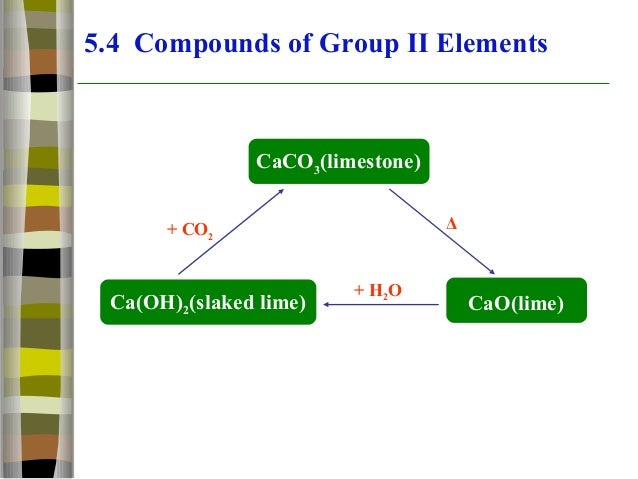
8.Write
the equations for the decomposition of calcium carbonate and magnesium
carbonate.
9.How
the thermal stability of nitrates and carbonates changes down
group 2
10.
How solubility and alkalinity of hydroxide
changes down the group2
11.
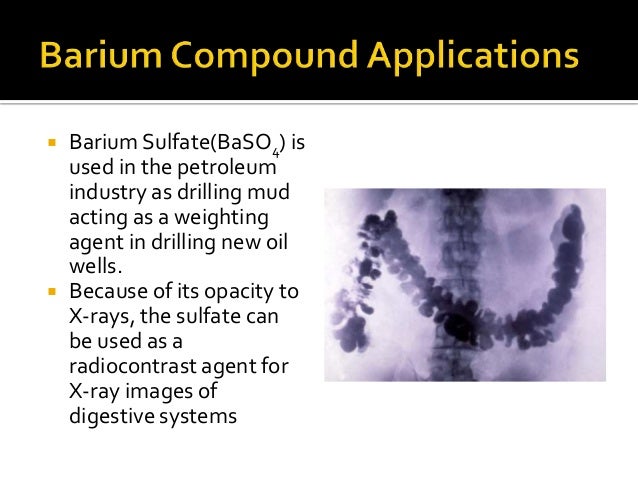
List the uses of Mg O , CaCO3 & BaSO4
12.
Explain the trends of the following properties across a period
a.
electro negativity
b.
atomic radius.
13.Explain
the trend in the following graph of atomic radius and atomic number.

.








 ..
..






























No comments:
Post a Comment