
Resources on other websites:
http://www.chemguide.co.uk/organicprops/carbonyls/background.html
https://www.khanacademy.org/science/organic-chemistry/aldehydes-ketones
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/aldket1.htm
www.youtube.com/watch?v=iOeDDme-Tl0
.


















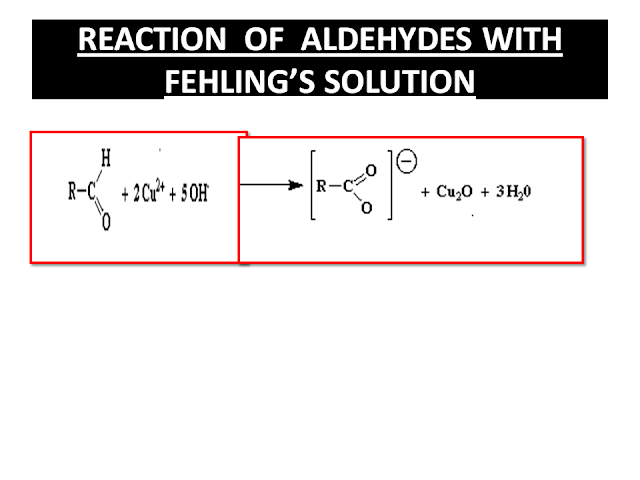















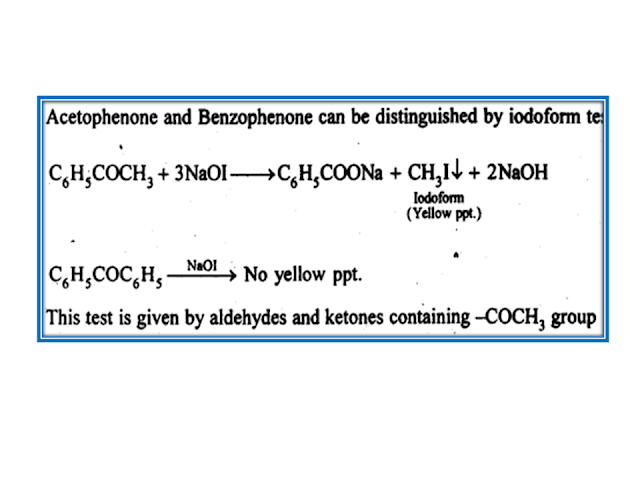


No comments:
Post a Comment