Fast and slow reactions
Some reactions are slower than others. For
example:
example:
- Rusting is a slow reaction
- Burning and explosions are very fast reactions
MEASURING REACTIONS INVOLVING COLOUR CHANGES
CONDUCTIVITY MEASUREMENTS.

TITRATION

Graphs
·
The rate of reaction can be analysed by plotting graph of amount of product against time. The
graph below shows this for two reactions.

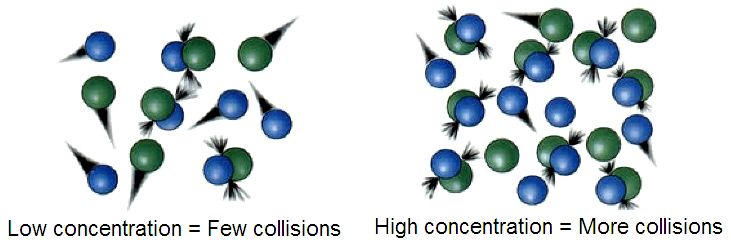
Collision theory
Effect of catalysts
CATALYTIC CONVERTERS
MAXWELL'S -BOLZMANN DISTRIBUTION OF ENERGY

MAX WELL BOLTZMANN DISTRIBUTION CURVE
ENZYMES
Enzymes and temperature..

ENZYME ACTIVITY
Everyday uses of enzymes..
Biological washing powders
·



Lactose intolerance


http://www.bbc.co.uk/schools/gcsebitesize/science/add_ocr_pre_2011/chemical_synthesis/ratereactionrev2.shtml
http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Factors_That_Affect_Reaction_Rates#Concentration_Effects
https://www.cdli.ca/sampleResources/chem3202/unit01_org01_ilo03/b_activity.html
http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Factors_That_Affect_Reaction_Rates#Concentration_Effects
https://www.cdli.ca/sampleResources/chem3202/unit01_org01_ilo03/b_activity.html




























No comments:
Post a Comment