PROPERTIES
Most
covalent compounds have relatively low
melting points and boiling points.
Covalent
compounds usually have lower enthalpies of fusion and vaporization than ionic
compounds.

Examples of dot and cross models
Molecule
|
Dot and cross model
|
Hydrogen (H2)
|
 |
Chlorine (Cl2)
|
 |
FORMATION OF METHANE (SINGLE)
.

FORMATION OF CARBON DIOXIDE (DOUBLE )

FORMATION OF OXYGEN

FORMATION OF NITROGEN MOLECULE (TRIPLE )

COORDINATE COVALENT BOND OR DATIVE BOND


The formation of the hydronium ion when the
water reacts with proton is also an example of
the co-ordinate bonding
water reacts with proton is also an example of
the co-ordinate bonding
H2O + H+ → H3O+

Valence Shell Electron Pair Repulsion Theory
 |
| Sir Ronald sydney Nyholm |
.
The five important electron-pair geometries are

SHAPES OF MOLECULES
GIANT COVALENT SUBSTANCES
1.Mention the shape of SF6 and draw the shape.
2.Define van der Waals' forces of attraction.
Diamond


FULLERENES
.


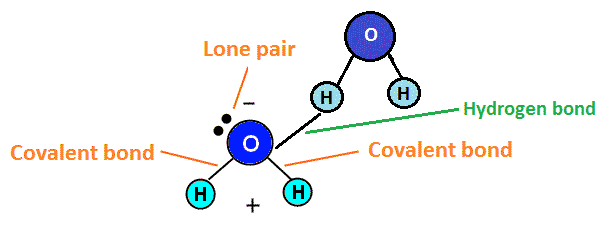
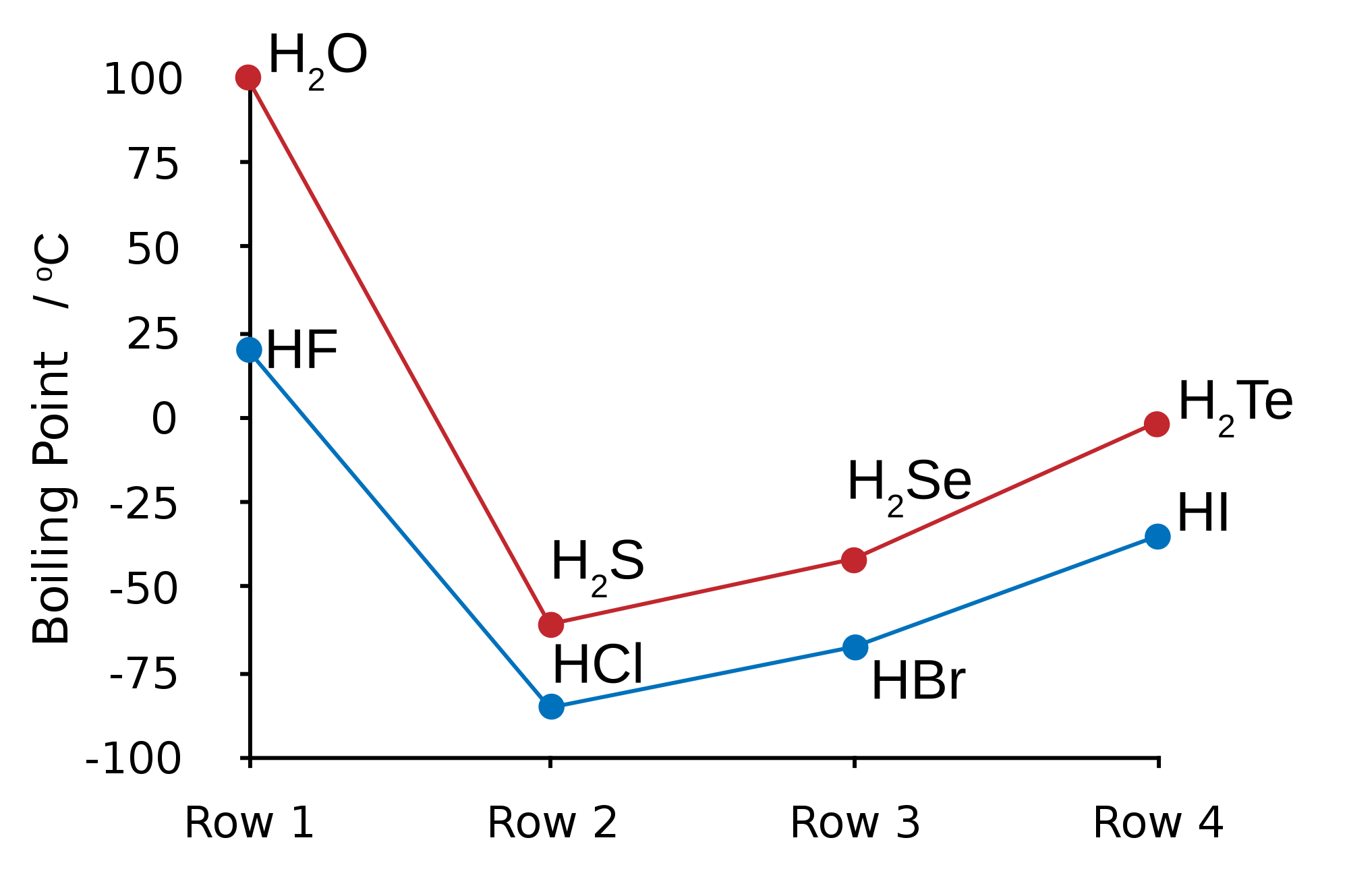
HYDROGEN BONDING IN WATER
HYDROGEN BONDING IN HF
HYDROGEN BONDING IN CARBOXYLIC ACIDS
HYDROGEN BONDING IN DNA
covalent bonding
http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds


































.jpg)














It has been simply incredibly generous with you to provide openly what exactly many individuals would’ve marketed for an eBook to end up making some cash for their end, primarily given that you could have tried it in the event you wanted.dental clinic near me
ReplyDelete