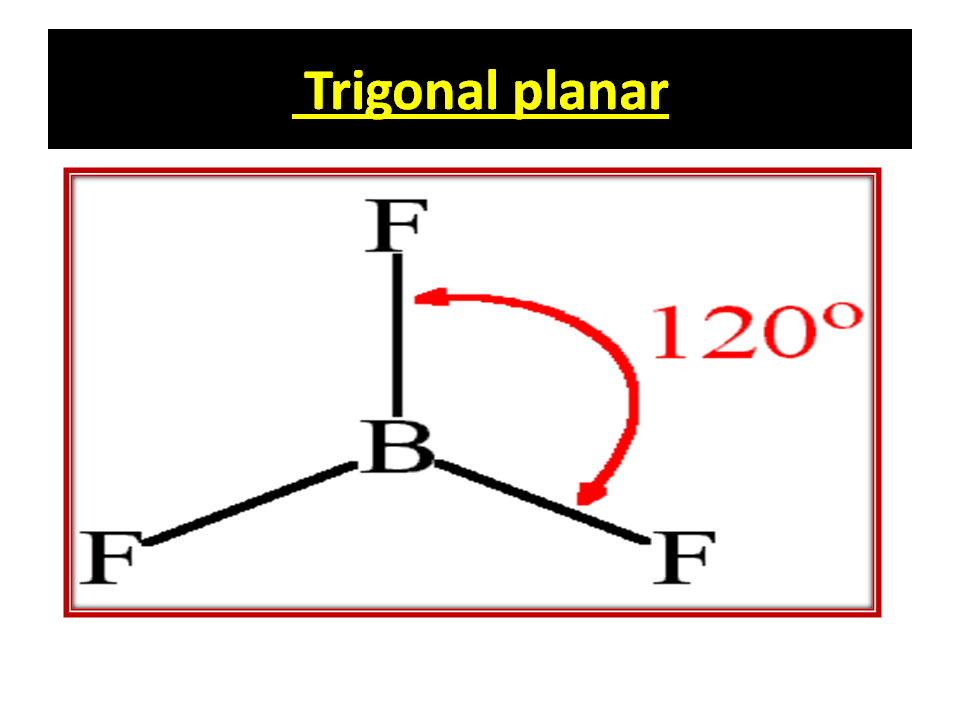
BORON TRI FLUORIDE
METHANE
AMMONIA
QUESTIONS
1.Give reason. The shape of water molecule is bent or angular.
2. Draw the structure of PCl5
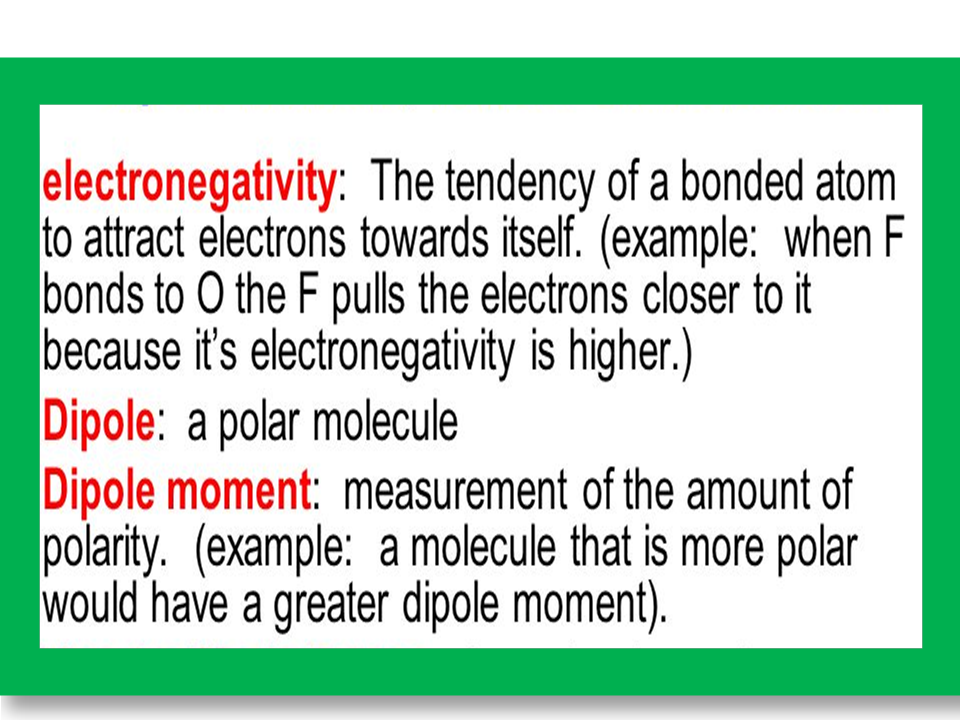
POLAR COMPOUNDS
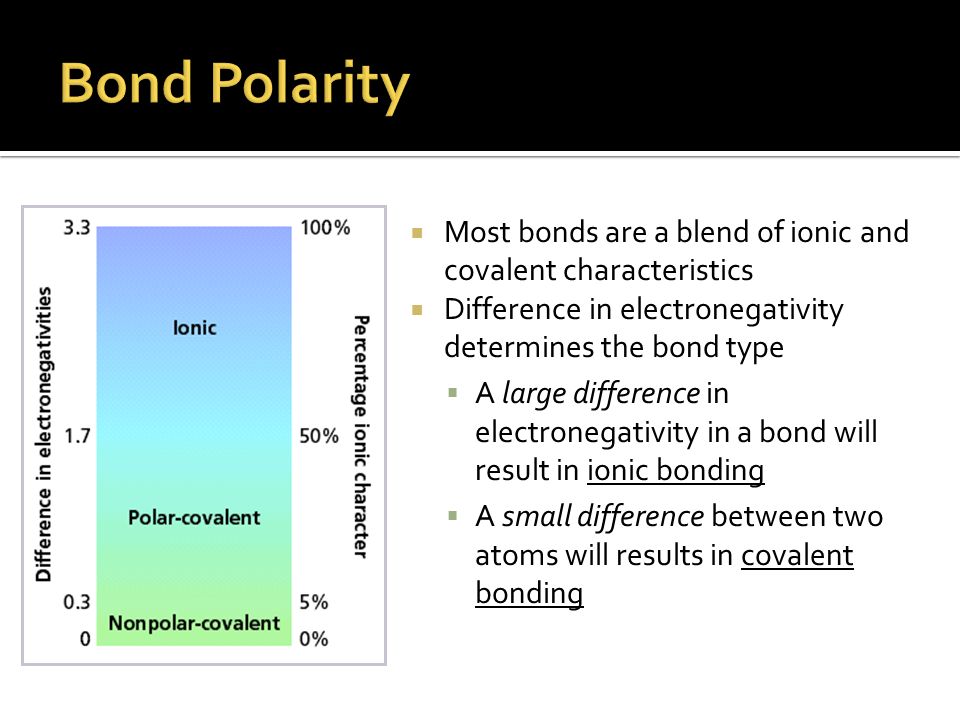
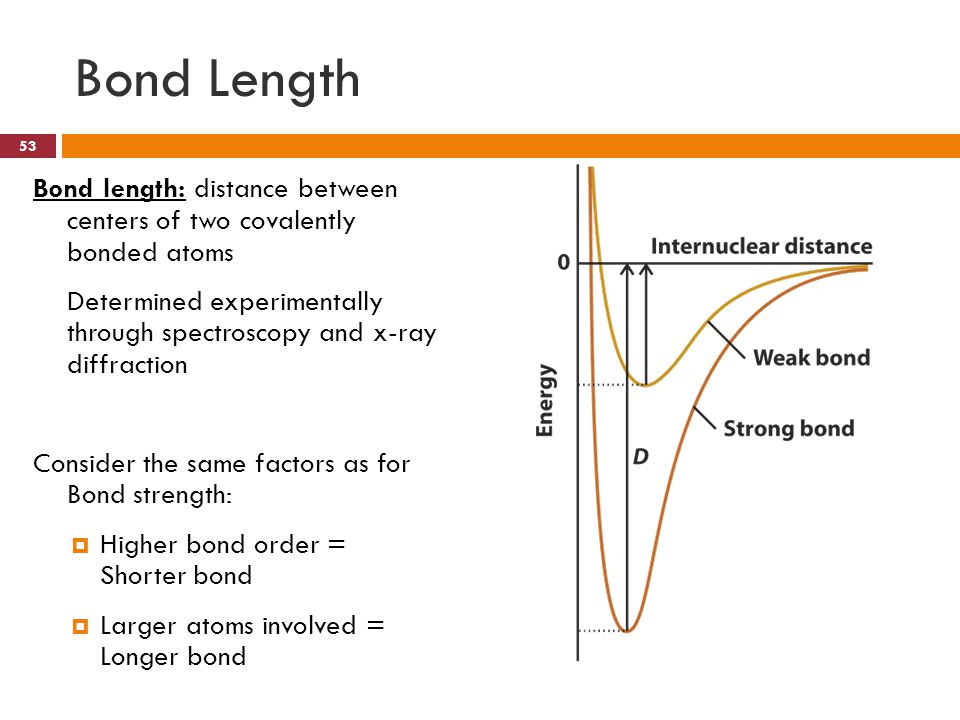

QUESTIONS
1.Which of the following have permenent dipole moment.
HCl, HBr, HF, Cl2, I2

LINK Hydrogen bonding
https://www.youtube.com/watch?v=keHS-CASZfc




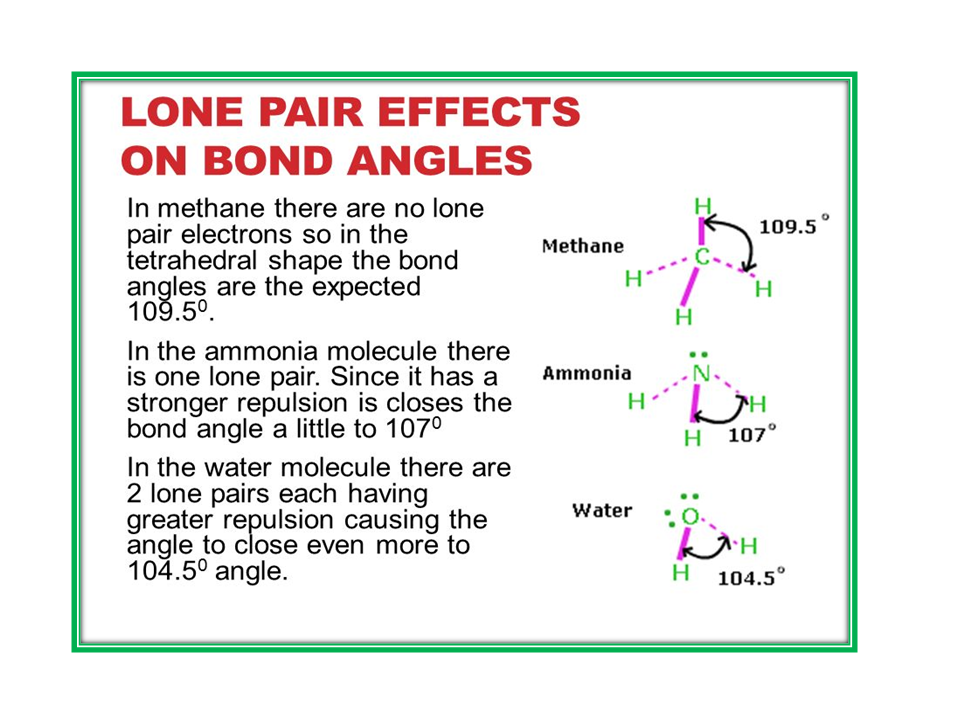





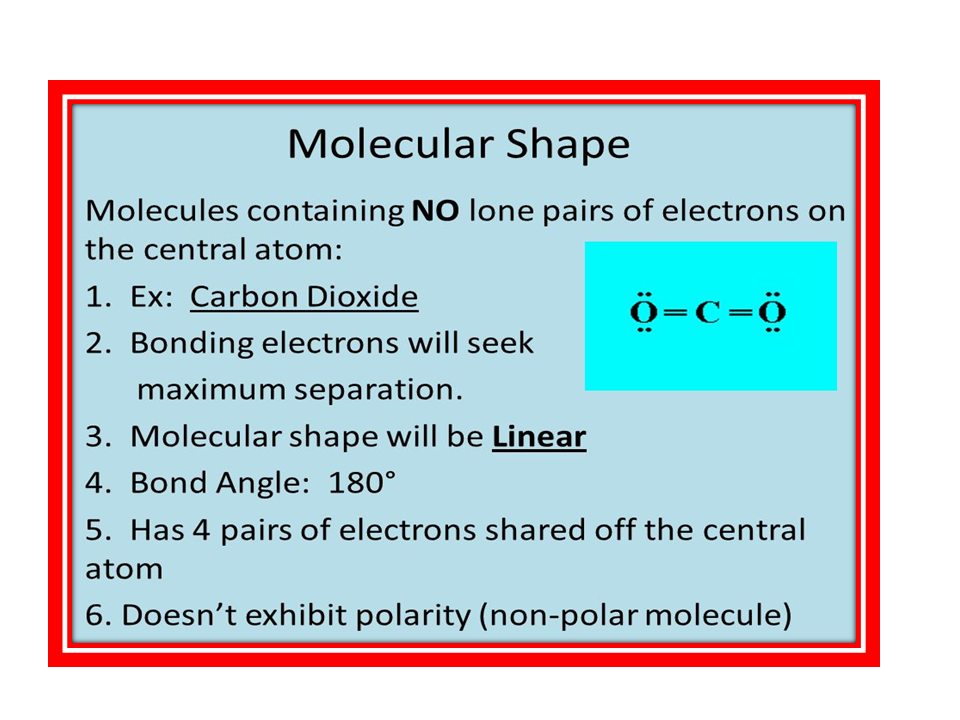















































No comments:
Post a Comment