
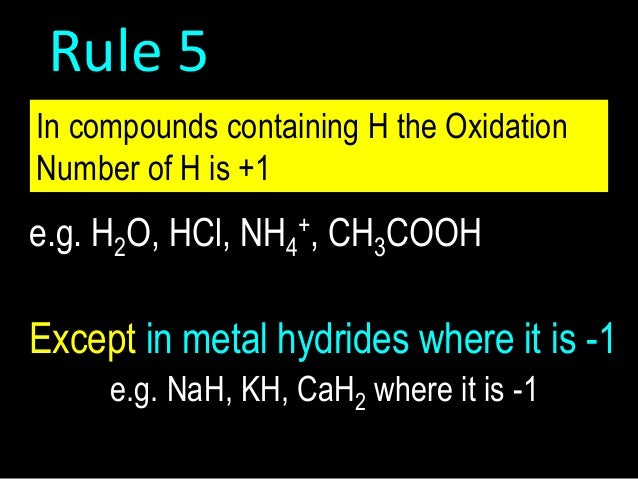
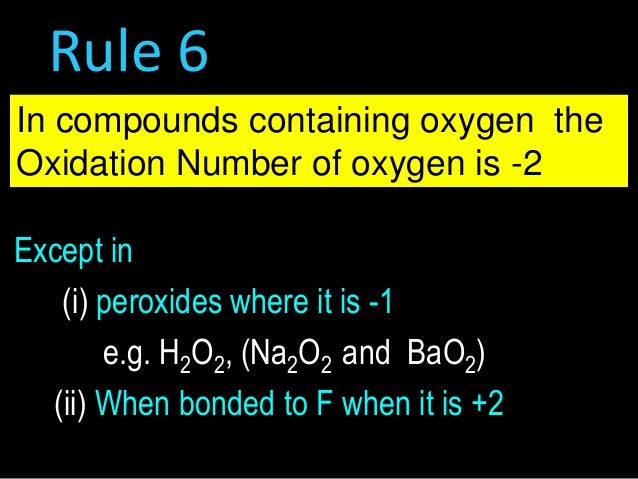
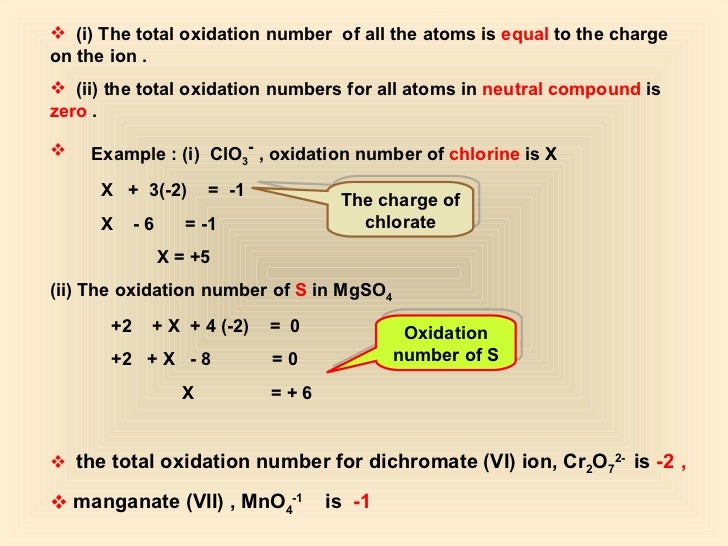
OXIDATION NUMBER CALCULATION
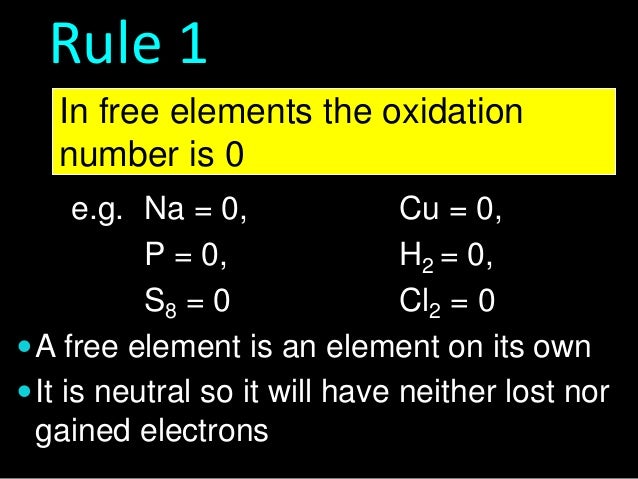
![Rule 4
The sum of oxidation numbers in a
complex ion is the charge on the ion
2-
SO4 = -2 [total]
PO43- = -3
NH4+ = +1
NO...](http://image.slidesharecdn.com/2-140309055203-phpapp01/95/261-oxidation-numbers-7-638.jpg?cb=1394344502)
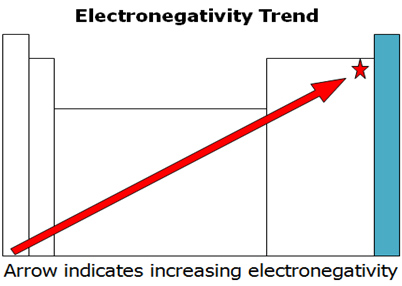
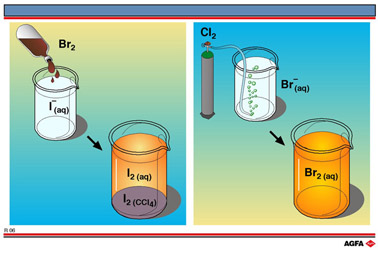
Oxidation States of Halogens
Halogen
|
Oxidation States in Compounds
|
Fluorine
|
(always) -1* |
Chlorine
|
-1, +1, +3, +5, +7 |
Bromine
|
-1, +1, +3, +4, +5 |
Iodine
|
-1, +1,+5, +7 |
Astatine
|
-1, +1, +3, +5, +7 |
BLEACHING PROPERTY OF CHLORINE
Chlorine is the
basis for the most commonly used bleaches, for example, the solution of sodium hypochlorite


.






































No comments:
Post a Comment