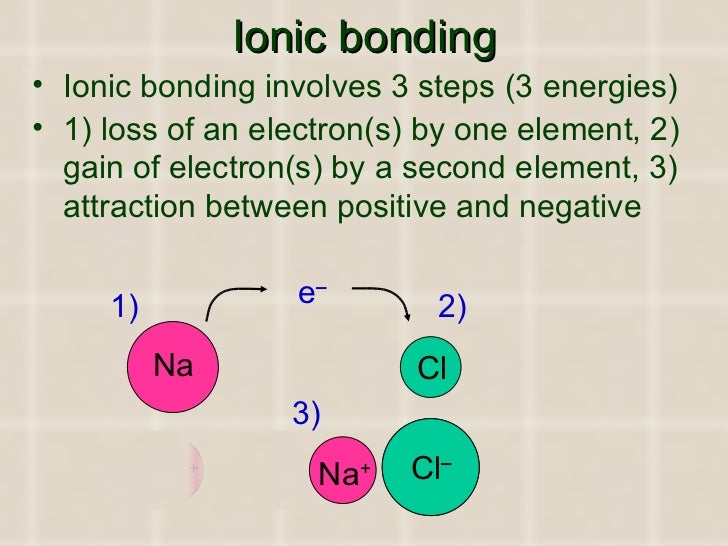
ELECTRON TRANSFER




· Metal atoms lose the electron, or electrons, in their highest energy level and become positively charged ions

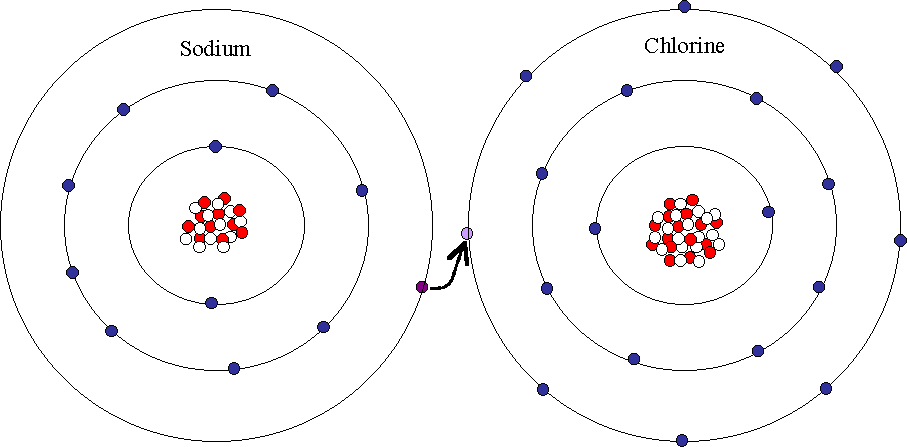

FLUORINE

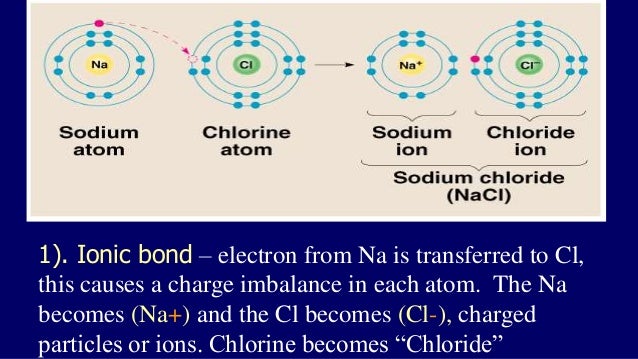
FORMATION OF NaCl:


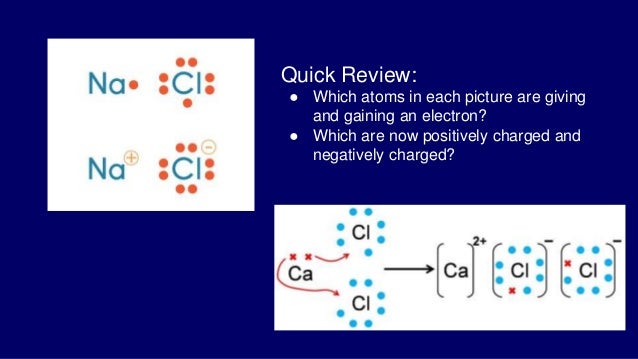
FORMATION OF CALCIUM CHLORIDE

FORMULA OF IONIC COMPOUNDS:
| Elements | Compound Formula | Compound Name | |
| Mg | Br | MgBr2 | magnesium bromide |
| K | S | K2S | potassium sulfide |
| Cl | Al | AlCl3 | aluminum chloride |
| S | Cu (+1, +2) | Cu2S | copper(I) sulfide |
| CuS | copper(II) sulfide | ||
| F | Zn (+2) | ZnF2 | zinc fluoride |
| O | Co (+2, +3) | CoO | cobalt(II) oxide |
| Co2O3 | cobalt(III) oxide | ||
| aluminum | oxygen | Al2O3 | aluminum oxide |
| calcium | iodine | CaI2 | calcium iodide |
POSITIVE AND NEGATIVE IONS
Name
|
Formula
|
Name
|
Formula
|
Name
|
Formula
|
ammonium
|
NH 4 +
|
magnesium
|
Mg 2 +
|
zinc
|
Zn 2+
|
hydrogen
|
H+
|
calcium
|
Ca 2 +
|
lead
|
P b 2 +
|
lithium
|
Li+
|
barium
|
Ba 2 +
|
iron(II)
|
Fe 2 +
|
sodium
|
Na+
|
silver
|
Ag+
|
iron(III)
|
Fe 3 +
|
potassium
|
K+
|
copper(II)
|
Cu 2 +
|
aluminium
|
Al 3 +
|
|
|||||||||||||||||||||||||||||||||||||||||||||
ALUMINIUM OXIDE

LITHIUM OXIDE

CALCIUM
CHLORIDE

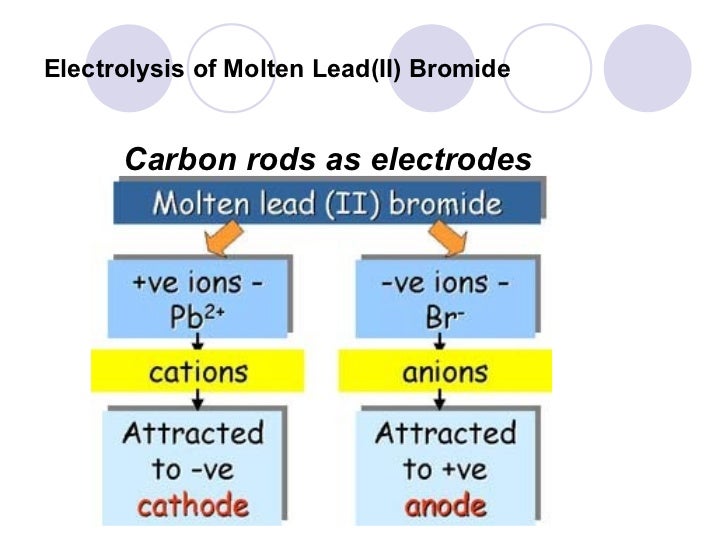

Solubility of ionic compounds in water.

IONIC BOND FORMATION
GIANT LATTICES OF IONIC COMPOUND
PROPERTIES OF IONIC COMPOUNDS


USES OF SODIUM CHLORIDE
—
USES OF SODIUM FLOURIDE

| magnesium oxide board. |

http://chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Ionic_Bonds
http://www.bbc.co.uk/schools/gcsebitesize/science/add_gateway_pre_2011/periodictable/ionicbondingrev1.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_gateway_pre_2011/periodictable/ionicbondingrev1.shtml



























No comments:
Post a Comment